
Top Performing Drug – Entyvio (February Edition)
Shots:
-
In continuation of our previous series on the Top-Performing Drug of the month, based on 2021 revenue, this month we have selected Entyvio and prepared a curated analysis report for our readers
-
Entyvio works on the mechanism of an integrin receptor antagonist, which is approved for moderate to severe active ulcerative colitis and active Crohn’s disease in adults.
-
PharmaShots presents a concise take on the key features of Entyvio with a detailed analysis of its revenue, clinical trials, alternatives, and approvals. The report is concluded with an engaging SWOT analysis and informative KOL reviews
Active Ingredient: Vedolizumab
Dosage Forms & Strengths:
-
Injection (IV): 300 mg vedolizumab in a single-dose vial
-
Injection (SC): 108 mg/0.68 mL solution in a single-dose prefilled syringe with needle safety device
-
Injection (ENTYVIO PEN): 108 mg/0.68 mL solution in a single-dose prefilled pen
Mechanism of Action: Integrin receptor antagonist
Originator: Takeda Pharmaceuticals
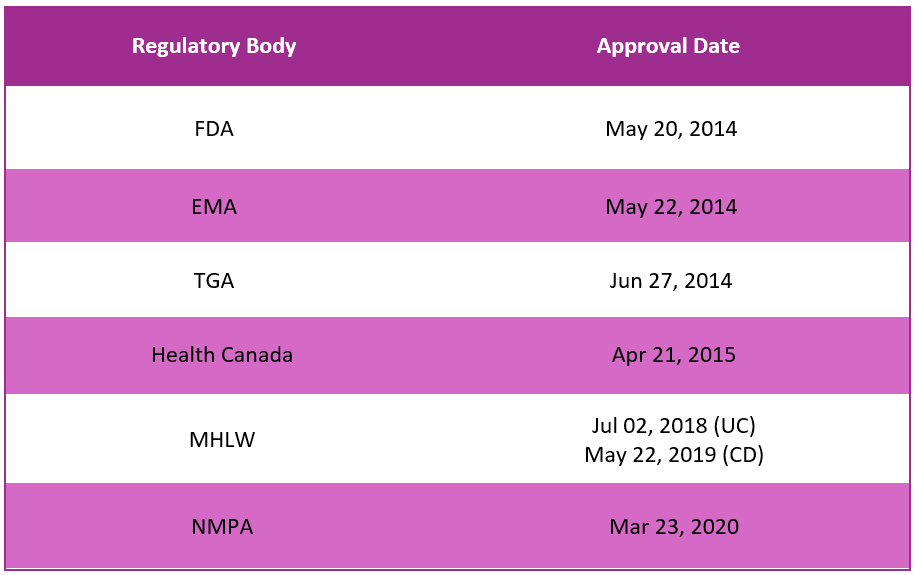
First approvals1,2,3,4,5,6
The table below depicts the first approvals of Entyvio by different regulatory agencies.
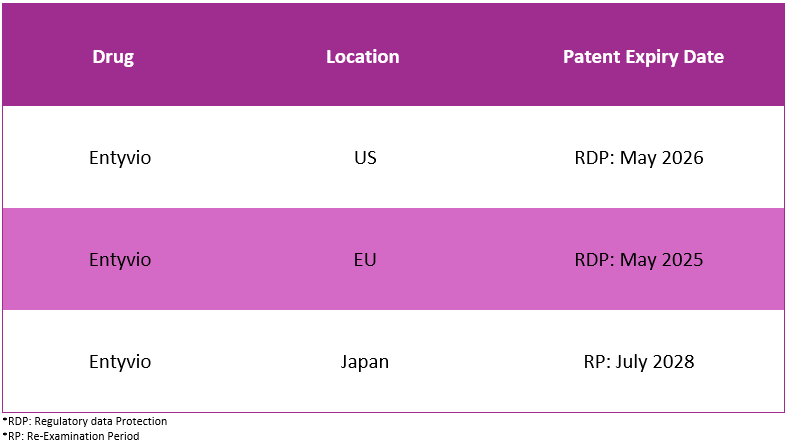
Patent7
The table below showcases Entyvio’s patent expiry in different geographies:
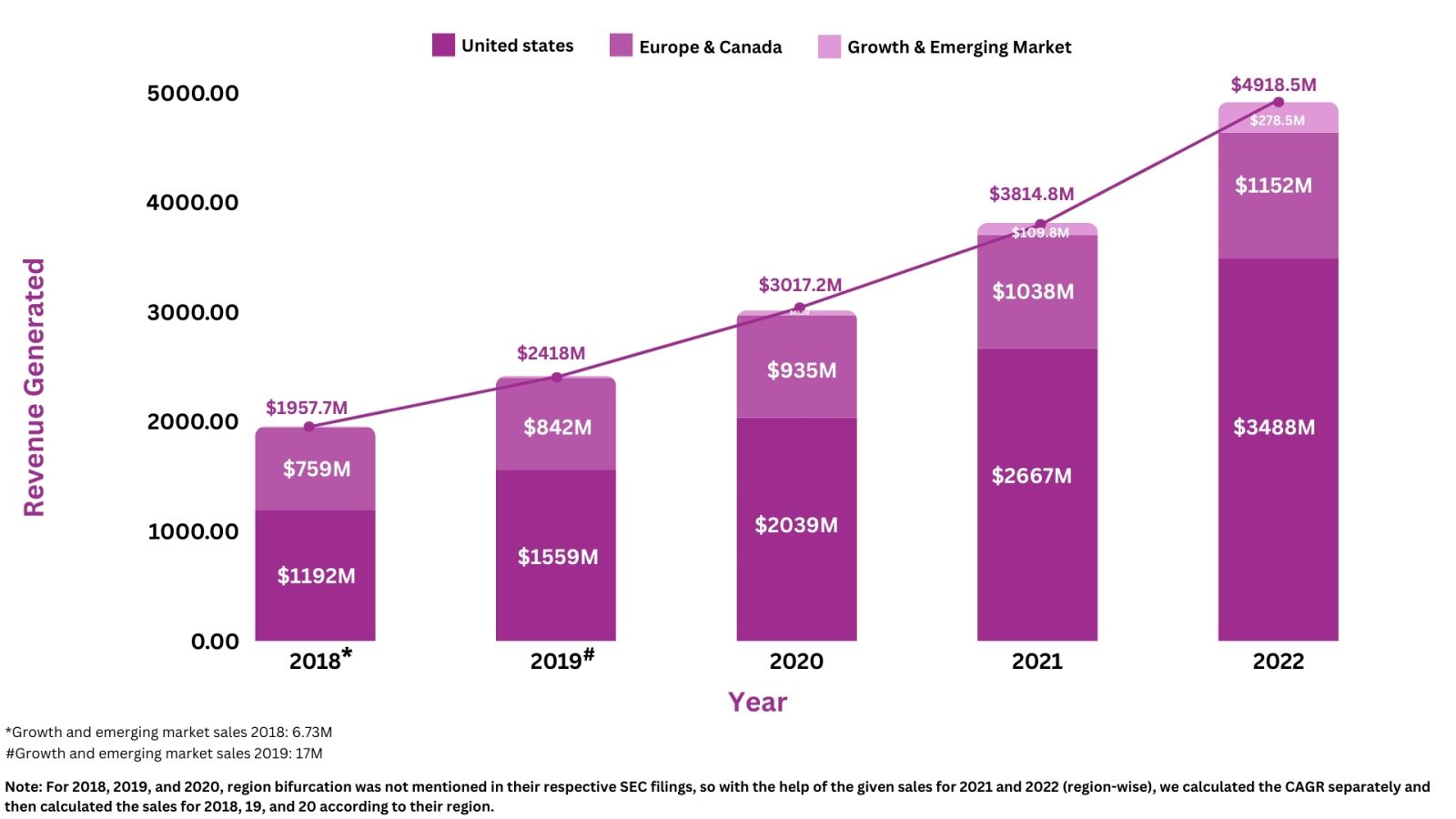
Revenue Analysis7
The annual sales of Takeda’s lead assets Entyvio, have substantially grown to add a huge amount of profit to the company’s overall revenue. In 2022, Entyvio generated total sales of $5,364M, representing a 20.8% increase from 2021. Over 5 years, the highest percentage change in the product’s overall revenue was seen in 2022, with a 20.8% increase in sales as compared to 2021. The rise in the product's revenues was attributed to increased demand among patients suffering from IBD. Moreover, in 2022, from the worldwide revenue of Entyvio, i.e., ~$5,292M, the US had the biggest share, contributing ~$3,704M, and then Europe and Canada had a combined share of $1,024M, and growth and emerging market had a contributed share of ~$278.52M.
The following graph illustrates the revenue analysis for the last five years' sales of Entyvio:
Approved Indications9
Entyvio is an integrin receptor antagonist is indicated for:
-
Moderate to severe active Ulcerative Colitis and active Crohn's disease (US)
-
Moderate to severe active Ulcerative Colitis and active Crohn's disease and Pouchitis (EU)
-
Moderate to severe active Ulcerative Colitis and active Crohn's disease (treatment & maintenance therapy) (Japan)
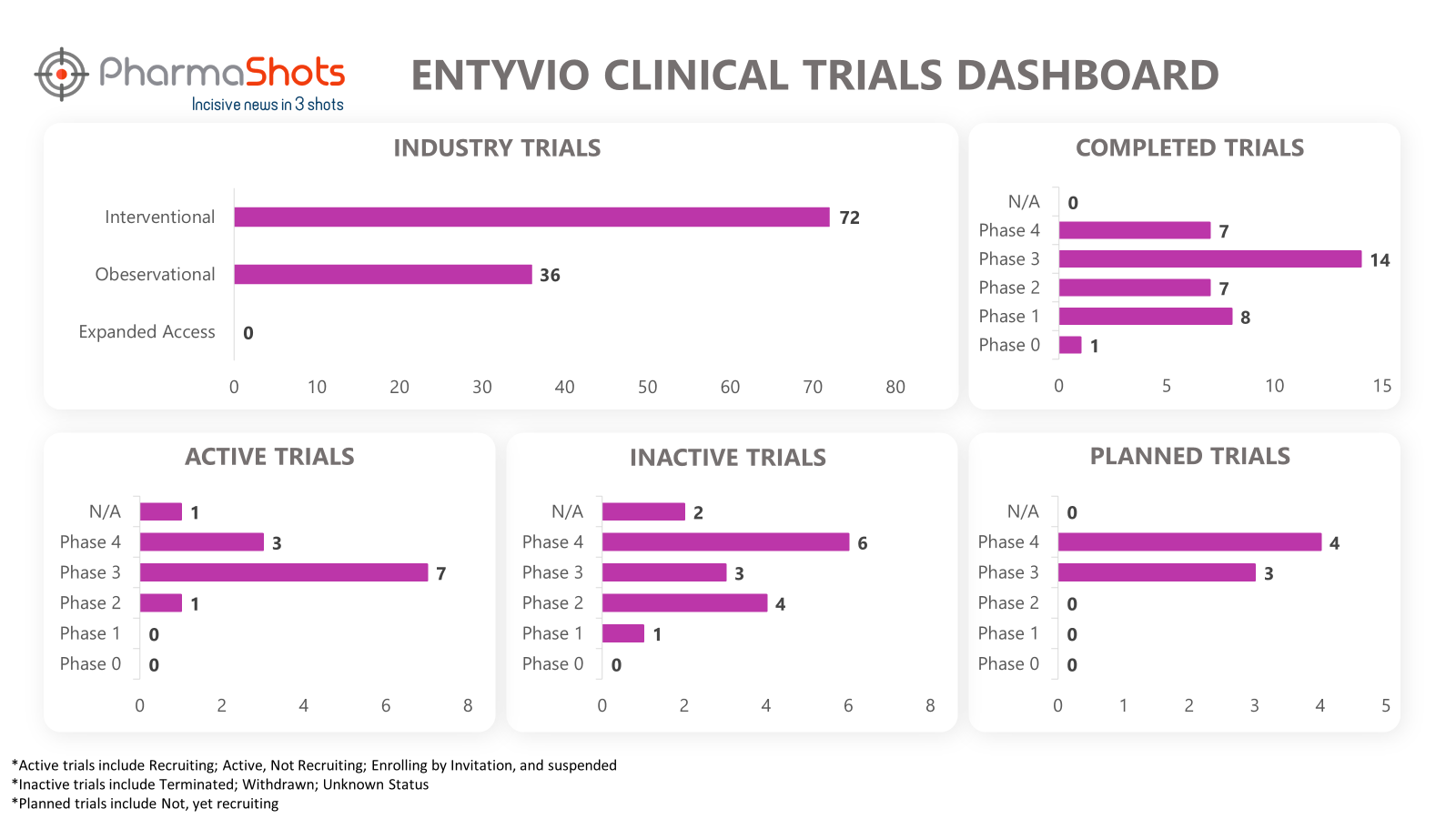
Clinical Trials Analysis8
Clinical trial analysis is vital for advancing medical science, improving patient care, and making informed decisions about the safety and efficacy of new treatments. It underpins the foundation of evidence-based medicine and regulatory approval processes, leading to better healthcare outcomes and the development of innovative therapies.
The following dashboard illustrates the clinical trials associated with Entyvio:
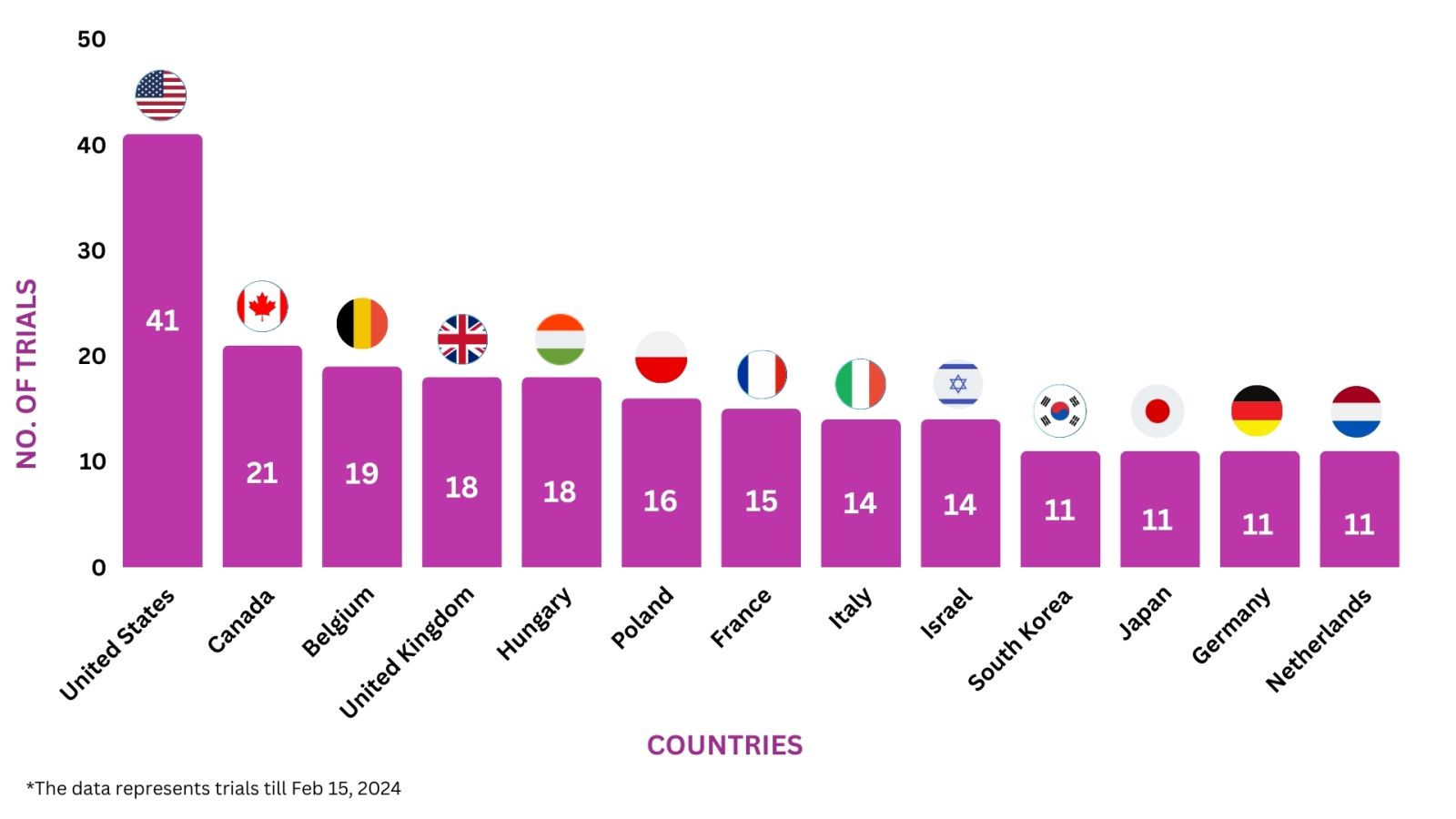
Entyvio Trials Representation (Country-wise)8
Below graphs depict ongoing trials investigating Entyvio:-
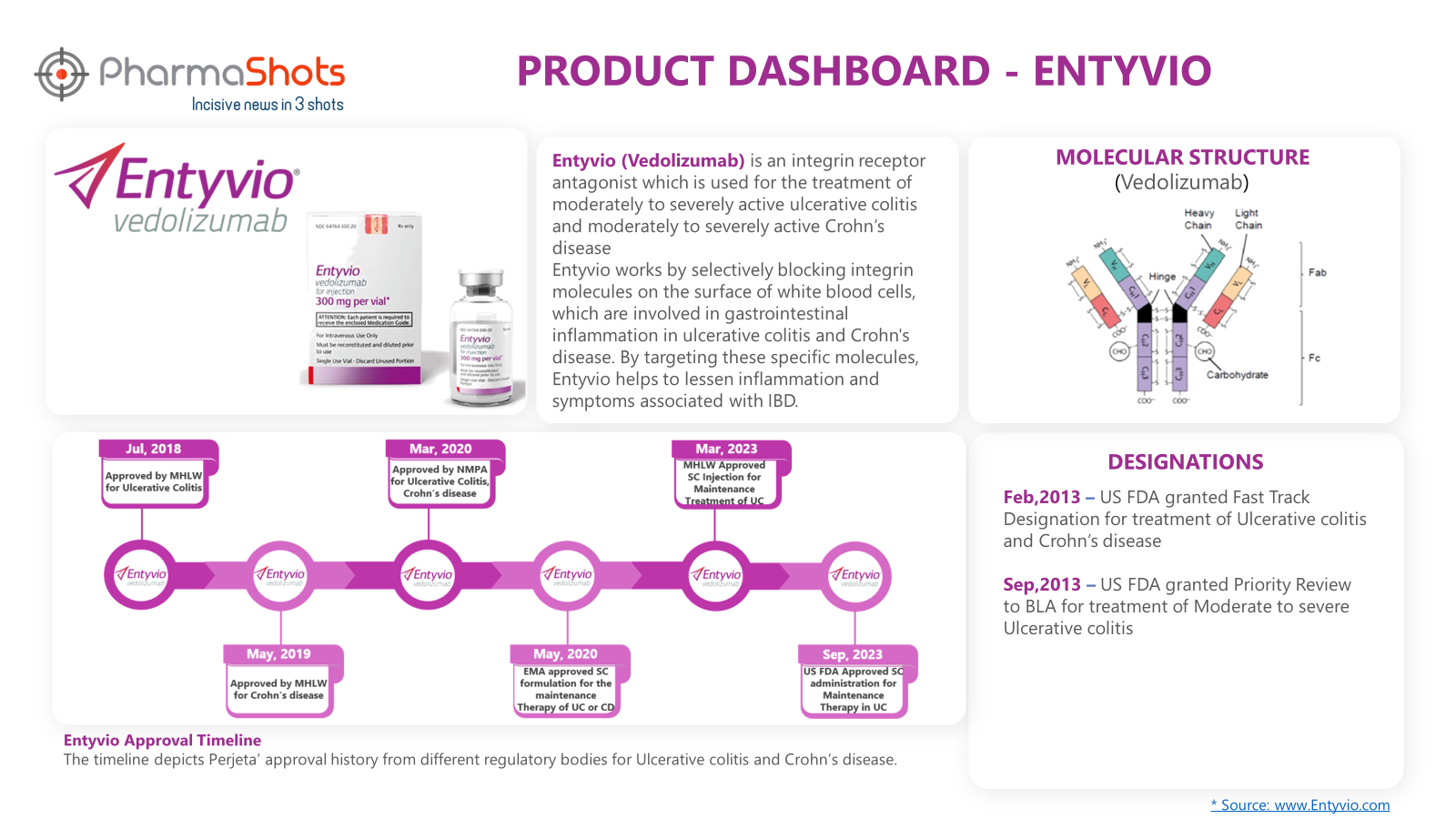
Product Dashboard9
PharmaShots presents an illustrative dashboard, highlighting essential metrics and pertinent information about Entyvio:
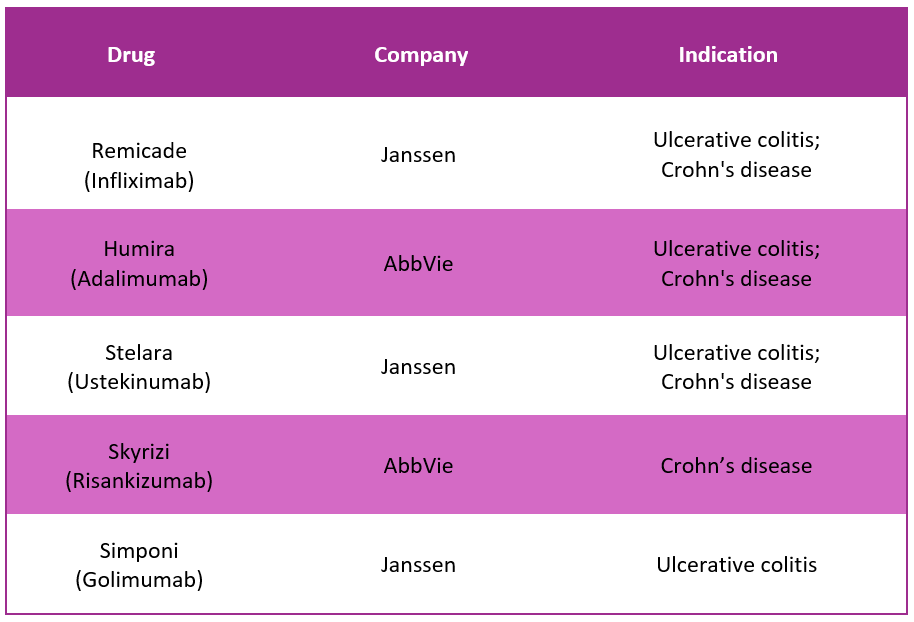
Entyvio Alternative Drugs10
In response to Entyvio, several alternative drugs are available in the market to treat Ulcerative Colitis and active Crohn's disease. Some of the substitute drugs for Entyvio include:
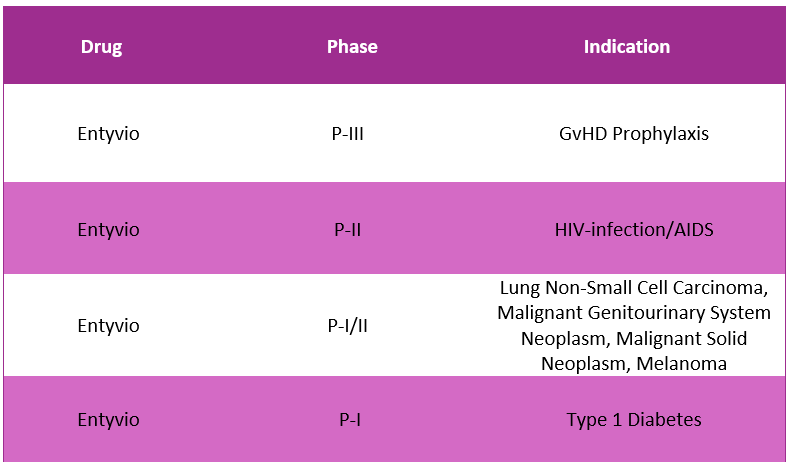
Entyvio Pipeline Analysis8, 11
PharmaShots presents an extensive analysis of Entyvio’s pipeline, including the ongoing P-I, P-II and P-III studies for various indications.
The table below depicts an overview of these studies:
Entyvio SWOT Analysis
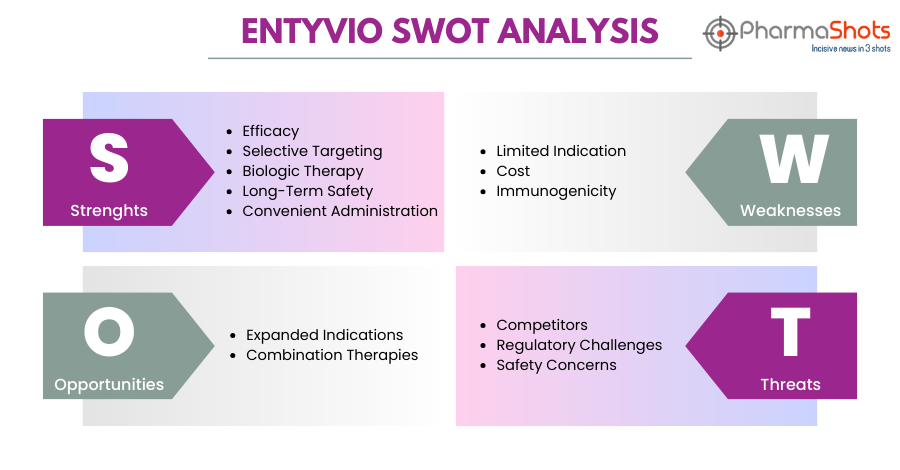 Strengths:
Strengths:
-
Efficacy: In Ulcerative colitis and Crohn's disease patients, Entyvio has shown effectiveness in inducing and maintaining remission
-
Selective Targeting: It targets integrin α4β7, decreasing inflammation in the stomach while not altering systemic immune responses
-
Biologic Therapy: Being a biologic therapy, it provides a therapeutic approach to the management of inflammatory bowel diseases and may lessen the side effects associated with systemic immunosuppression
-
Long-Term Safety: According to clinical research, Entyvio has shown a long-term safety profile with a reduced risk of serious infections than certain other biologic medicines
Weaknesses:
-
Limited Indication: As Entyvio is approved to treat moderate to severe ulcerative colitis and Crohn's disease which limits its use to specific patient populations
-
Cost: Biologic medicines, like Entyvio, can be expensive, which limits their accessibility to some patients
-
Immunogenicity: Like other biologics, Entyvio may cause an immunological reaction in certain people, which could result in a reduction in the medication's effectiveness or the formation of antibodies against it
Opportunities:
-
Expanded Indications: There might be chances to investigate Entyvio’s effectiveness in other autoimmune or inflammatory conditions beyond ulcerative colitis and Crohn's disease
-
Combination Therapies: Examining Entyvio in combination with other therapies, including immunomodulators or corticosteroids, could improve its effectiveness in specific patient groups
Threats:
-
Competitors: Entyvio competes with existing biologic medicines and small molecule inhibitors for the treatment of ulcerative colitis and Crohn's disease
-
Regulatory Challenges: Changes in regulations or standards may impact Entyvio's market access and reimbursement
-
Safety Concerns: Any Entyvio-related safety concerns or unfavorable events could draw regulatory attention or result in a drop in the prescription rates
Patient Stories12, 13, 14
Patients' stories are the key resources as they provide a holistic perspective on the impact of medications on individuals' lives, improve healthcare decision-making, and contribute to the overall understanding of the drug's efficacy and safety in real-world settings. We have summarized some of the patients’ stories for Entyvio:
- SHEILA’S STORY: At the age of 20, she was diagnosed with Crohn's disease after a colonoscopy. Then she went from one medication to another for almost 8 years, after consulting with her doctor they recommended Entyvio, and over time, she started showing improvement
- SARAH’S STORY: Sarah was diagnosed with severe ulcerative colitis; at first, she thought it might have been something she ate or something she drank, but as symptoms worsened, she went to a GI specialist and was diagnosed with ulcerative colitis. Her GI recommended Entyvio. Since she started Entyvio, she noticed that her symptoms are beginning to improve
- MAMIE’S STORY: 8 years ago, Mamie was diagnosed with severe Crohn's disease. She then started on various medications but none of them helped with symptoms for more than a few months. She then consulted another GI doctor who recommended Entyvio and after a while, she began to show improvement
KOL* Reviews15, 16, 17, 18, 19
1. Bruce Sands, M.D., M.S., Chief at the Icahn School of Medicine at Mount Sinai.
“The VISIBLE 1 trial demonstrated that ENTYVIO SC can provide physicians with an additional administration option for achieving remission in their moderate to severe ulcerative colitis patients. Since its approval in 2014, ENTYVIO has continued to build a robust safety and efficacy profile. I appreciate now having a subcutaneous administration option that provides a clinical profile consistent with ENTYVIO IV while also giving me and my appropriate UC patients a choice of how they receive their maintenance therapy.”
2. Dr. Brian Bressler, Director at the University of British Columbia.
"Having a formulation of vedolizumab that can be self-administered is an important new delivery method for IBD patients with Crohn's disease or ulcerative colitis. These diseases are lifelong conditions so giving patients choices is helpful when it comes to managing this disease.”
3. Brandon Monk, Snr. Vice President, Head, U.S. Gastroenterology Business Unit, Takeda
“With the FDA approval of subcutaneous ENTYVIO, patients and physicians who want ENTYVIO’s clinical profile along with flexibility of administration now have two choices for maintenance treatment for adults with moderate to severe ulcerative colitis, Takeda is committed to meeting the varied medical needs, circumstances and personal preferences of people living with UC as they progress in their lifelong journey with the disease. ENTYVIO is the only FDA-approved biologic for maintenance therapy in ulcerative colitis offering the option of either intravenous or subcutaneous administration.”
4. Vijay Yajnik, M.D., Ph.D., Vice President, Head of U.S. Medical for Gastroenterology, Takeda
“With two applications for a subcutaneous option of ENTYVIO now under FDA review, we remain firm in our commitment to the inflammatory bowel disease community—adults with ulcerative colitis or Crohn’s disease—and the health care professionals actively managing their care. Every patient’s journey is different, and every patient has a unique set of medical needs and personal preferences. We strongly believe in meeting those needs—providing both an IV and a subcutaneous administration option for ENTYVIO, pending approval, is one way we can do that.”
5. Dr. Jefferson Tea, Vice-President Medical & Scientific Affairs, Takeda Canada
"Takeda is committed to continued innovation in IBD to bring novel solutions to meet the diverse needs of the patients we serve. We put the health and wellbeing of patients first and support them through different programs, ongoing research and by advancing science to give patients options that reflect their lifestyle. The availability of this new formulation of ENTYVIO® marks an important milestone in our continued commitment."
* Key Opinion Leaders (KOLs) are crucial when it comes to the launch and assessment of pharmaceutical products. At Octavus, we recognize the importance of KOLs in the industry, which is why our proficient team dedicatedly tracks their activities and provides valuable insights to the pharma fraternity.
We understand that KOL tracking and selection can be overwhelming and time-consuming. That's why we offer extensive KOL tracking services to help our clients stay ahead of the curve. Our team of experts can provide you with the latest information on KOL activities, including their opinions, publications, and affiliations.
Interested in learning more about our KOL tracking services? Don't hesitate to reach out to us at bd@octavusconsulting.com or connect@pharmashots.com. We would be more than happy to provide you with more information and discuss how our services may benefit your business.
Octavus is a dedicated consulting company that offers a one-stop market solution to life science enterprises, biopharma, MedTech, diagnostic centers, digital health companies, animal healthcare, and start-ups.
References:
- Drugs.Com
- EMA
- TGA PI
- Health Canada
- MHLW
- NMPA
- Takeda Sec filings
- ClinicalTrials.Gov
- Entyvio
- Mayo clinic
- Takeda Pipeline
- Sheila Story
- Sarah Story
- Mamie Story
- KOL (Bruce Sands)
- KOL (Brian Bressler)
- KOL (Brandon Monk)
- KOL (Vijay Yajnik)
- KOL (Dr. Jefferson Tea)
Related Post: Top Performing Drug – Perjeta (January Edition)

Disha was a content writer at PharmaShots. She is passionate and curious about recent updates and developments in MedTech and Pharma industry. She covers news related to clinical trial results and updates. She can be contacted at connect@pharmashots.com.














